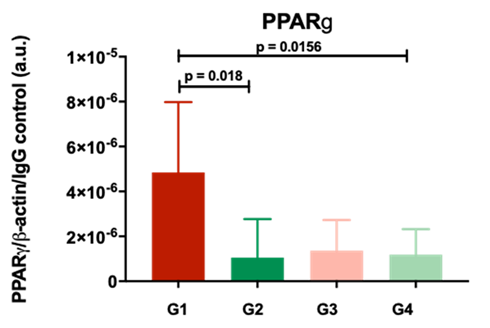For this edition of the QUOD newsletter, the spotlight shone on Dr Bill Scott who is the Scientific Director of Transplantation and Regenerative Medicine at Newcastle University, liaising with colleagues in all transplant specialities, orchestrating the daily running of the labs with a focus on moving basic and translational research into clinical practice. Bill is a key member of the QUOD hub in Newcastle, formed during the MRC funded expansion of QUOD to include whole organs made available for research. This important steppingstone will form the basis of many more research projects and enable access to bespoke samples to help answer burgeoning questions posed by the wider transplant community.

Bill has spoken of his interest in improving access to tissue for research purposes, which has led to his collaboration with QUOD. He commented on how QUOD is uniquely positioned to address fundamental questions by facilitating access to blood, urine and tissue samples which are intrinsically linked to details of the donor. When asked what he thought was the biggest hurdle facing sample accessibility, he highlighted the importance of effective communication between all parties concerning consent and regulation at the start of the retrieval process. “As we leverage new techniques” he said, “we need to consider how we communicate this [information] to a lay audience”.
Bill has many more strings to his bow, which I was delighted to discuss with him. Originally hailing from the United States, Bill recalls his aptitude for maths at an early age, entering maths competitions when at school. He later undertook an undergraduate course in engineering at Ivy League University, Cornell, which also included core biology modules in addition to maths and engineering. These early studies underpinned his combined interest in medicine and engineering; after all, an engineer is adept at problem-solving, and throughout his postgraduate and doctoral training in Minneapolis in biomedical engineering, it became evident to him that the fundamentals of engineering could be applied to help solve problems in the clinical sphere. His interest was piqued by organ donation and transplantation research where there were, and still are, a number of issues to tackle, all with the common goal of helping clinicians and improving transplant outcomes for patients.
The NHS relies on companies making reasonably priced and accessible devices to help healthcare professionals deliver expert care to their patients. Bill highlighted the problem that many products are not affordable and are manufactured in the United States with the US reimbursement model, which differs completely from that of the UK and Europe. New devices developed on the back of high-quality research must serve real-world needs as well as make a profit for investors. Sounds like a tall order, right?
With this in mind, Bill took his years of research and learning a whole new vocabulary to approach and pitch to investors, sought to develop a device that could address key issues raised by the transplant community. At present, the demand for organ transplants far exceeds the supply, and current preservation systems rely on static cold storage which cannot be relied upon to keep the donated organ in optimal condition for more than a few hours. Methods that can sustain optimal conditions for prolonged periods of time are too large, complex and expensive. Bill, in collaboration with clinical engineers, software developers and investors, have developed the ScubaTx device designed to be fit for the real-world transplant environment and automate as much of the process as possible, while providing feedback on key events for the surgical team who can focus their attention on the transplant procedure rather than setting up a machine. Funds have been raised through Innovate UK and currently a number of prototypes are going to stakeholders across the world, with the hope that one day in the near future the device will be suitable for commercialisation.
Over the years, Bill has also successfully acquired financial backing for several start-ups and research projects. When asked what advice he would give to early career researchers and future entrepreneurs looking to write their own grant proposals, he emphasised the need to just put yourself out there. “You miss out on 100% of opportunities you don’t try for, [and] it takes 100 times of repeating the same task to get good at something” he said. Essentially, persistence and resilience are key. Bill also advised to “not limit yourself to one funder” and take time to understand what motivates the funder, as they will each want something different. He continues by describing the continual evolution of a grant proposal, adapting to the feedback received and the importance of finding the right investors and stakeholders who share your vision.
Bill can often be found in front of an audience of another kind as he leads the MRes Transplantation course as a Senior Lecturer and Associate Professor. He says it is a very rewarding experience as he gets the opportunity to inform, inspire and foster greater progress and interest in the field in the next generation of nurses, healthcare professionals and researchers, all of whom form the backbone of the NHS. For Bill personally, teaching also gives him the chance to re-connect with his specialist field, to see the bigger picture and highlight problems that face the current generation.
When asked what piece of advice he would give to his students and mentees, he highlighted the importance of forming a narrative, regardless of whether you are creating a poster, presentation or producing written work like a grant proposal or manuscript, “if you do not tell a story, then you are simply stringing together a series of facts on the screen or on paper, expecting others to read the situation exactly as you do”.
When Bill is not teaching, presenting or working in the lab, he enjoys hikes in the countryside with his dogs and fishing, where he can embrace the tranquillity and explore the natural beauty of Northumberland.
Dr Bill Scott was interviewed by Hannah McGivern