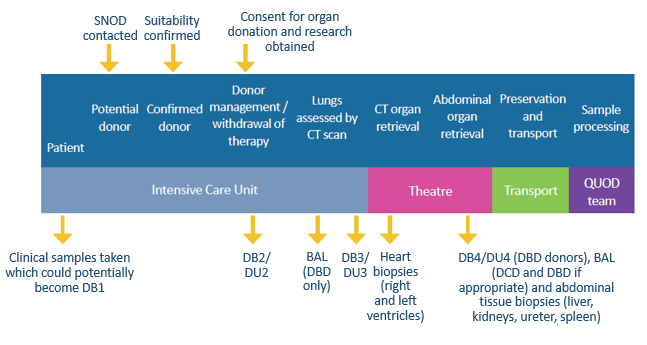
Bespoke, ergonomic and unique to the Quality in Organ Donation (QUOD) biobank, the QUOD sample collection boxes are critical to the transportation of donor blood, urine and tissue collected by nurses and retrieval teams. Sample collections are taken at four different time points across the donor management period, all the way through to the point of organ retrieval.

Manufacture
These custom-made sample collection boxes are assembled by hand and distributed nationally from the Oxford hub by QUOD Technical Assistant, Sophia Ali. Sophia joined the team four years ago and she continues to ensure that the Specialist Nurses in Organ Donation (SNODs) and retrieval teams across the UK are well-stocked with QUOD boxes, as well as other essential resources needed in theatre for sample collection. This can include biopsy punches and other equipment to aid in processing samples in the lab. “The most vital piece of kit which I am responsible for building and supplying across all hospitals in the UK”, she says, “is the famous QUOD box.” Watch a video demonstration of Sophia constructing a QUOD box [here] or read the step-by-step process from Sophia below.
How to build a QUOD box by Sophia Ali
“The QUOD box is made up of several components. You start with the outer box which is initially flat packed. Our vibrant QUOD sticker as well as the receipt and map sticker are applied. At the back of the box, we store the two information sheets, one for Scotland and one for England, Wales, and Northern Ireland along with the consent sticker.”
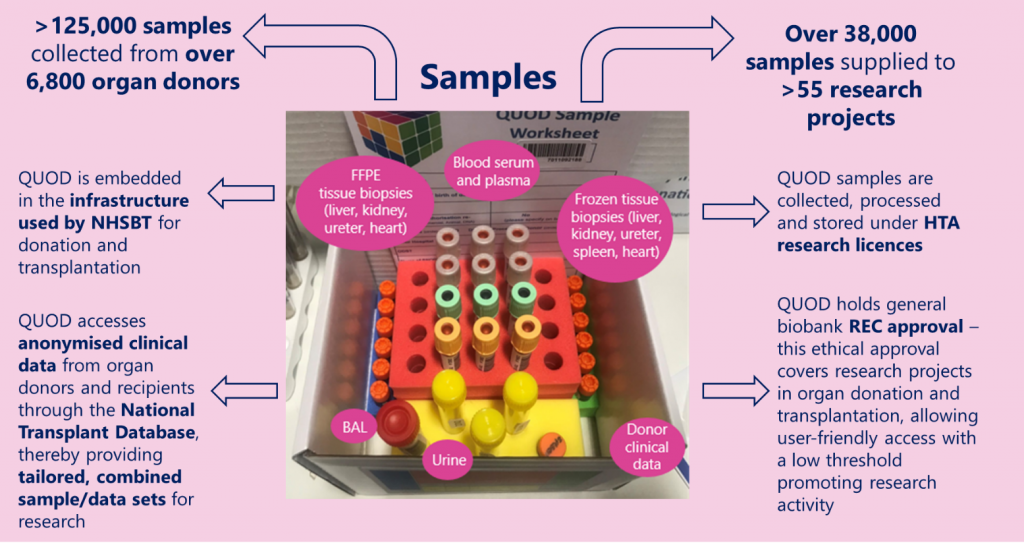
“Inside the box you will find individual red, green, blue, and yellow foams. Each foam represents a separate component and holds a different sample from the donor. The red foam will hold twelve blood tubes – six EDTA, three Heparin and three SST tubes. All are barcoded to identify the collection at separate time intervals. In the yellow foam you will find the BAL tube, three urine tubes and one (orange top) spleen container filled with RNAlater preservative. The green and blue foams contain the five tissue tubes. In the blue foam, the five tissue tubes are filled with formalin and the green, with RNAlater preservative. We currently collect liver, left kidney (in formalin), right kidney (in RNAlater), ureter, right heart ventricle and left heart ventricle. Each box will contain a worksheet where each sample collection can be recorded, and any variance notes can be added. There will also be DB1 and DB2 collection for blood serum in the preliminary stages of donation. Each box will be labelled with a unique barcode to ensure complete traceability. I also make note of where each box is shipped to via this barcode.”
Shipping
On a typical working day Sophia will respond to e-mail requests from SNODs and regional centres, subsequently building each QUOD box and packaging consumables accordingly, ready for shipping: “Due to demand, I have to make sure I have enough material to fulfil all requests. I aim to complete all requests within two working days”. During her time at QUOD, Sophia has calculated that she has built and distributed approximately 4,250 QUOD boxes since 2019! With the rising cost of consumables in recent years, the cost to produce and ship one QUOD box is currently around £60.00 GBP.
From retrieval to research
Tissue biopsies are stored in two different types of media for transportation to preserve the tissue in the best way possible whilst allowing for the widest variety of techniques to be carried out in future studies. Each sample has a unique identifying barcode which is recorded on the QUOD database to preserve traceability and donor anonymity throughout the whole process. The QUOD database links clinical donor information to these samples, allowing researchers access to samples that meet the requirements of their research.

Evolution
The current design represents years of continual evolution and improvement (as pictured), in accordance with changes to legislation from the early stages of QUOD to today. 2023 marks a ten-year milestone which we have honoured with a new logo that can be seen on each QUOD box.
Sophia remarks how the design is under constant review and has seen major changes in recent years, including changes to tissue collection and corresponding changes to the worksheet. Most recently being the change to kidney biopsies, whereby the biopsy from the left kidney goes into formalin, with the biopsy from the right kidney being preserved in RNAlater. Previously, biopsies from each kidney were divided in two, with each half going into formalin or RNAlater. There have also been changes to the sizes of heart and kidney biopsies over the years, in response to feedback from retrieval teams who collect the samples, researchers who use the samples and results from internal quality assurance audits.
A decade of QUOD marks a time of reflection for the whole team, as we look back on the evolution of the biobank over the last ten years and think about all the people who have helped us reach this landmark. We look forward to celebrating this achievement with our wider QUOD family throughout the year and most especially at our symposium on Friday 8th December 2023 at Exeter College in Oxford.











