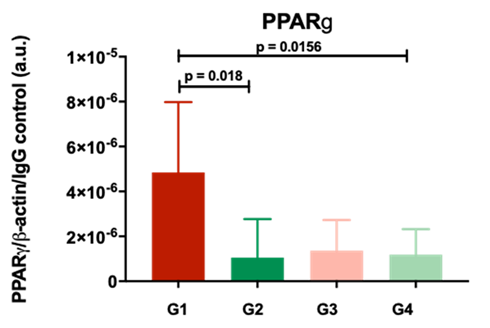Tobias Bohnenpoll, Evotec SE

Evotec SE and the University of Oxford have recently renewed their collaboration agreement, reflecting a robust and sustainable partnership to profile tissue biopsies collected, processed and stored by the QUOD programme. Through this partnership, Evotec has already generated genome-wide bulk transcriptomic data from about 2,000 donor biopsies (895 kidney and 910 liver), complementing the rich clinical records and technical metadata collected by NHSBT and QUOD. Evotec is currently extending the analysis to heart biopsies and high-resolution single cell and spatial transcriptomics, which will allow in-depth molecular characterisation of tissue microenvironments. Together, we aim to generate mechanistic insights to improve the quality of organ donation and transplantation, and to identify targetable mechanism of injury and repair that will open new avenues for therapeutic intervention and patient stratification in metabolic, cardiac and renal diseases.
The multimodal characterisation of organ biopsies using state-of-the-art, high-throughput analysis is an important cornerstone of Evotec’s Molecular Patient Databases (E.MPD), an industry-leading, proprietary collection of curated human datasets for translational research and patient-centric target and biomarker discovery. However, the integration of complex clinical and molecular data into curated multidimensional datasets poses great challenges to scientific teams and requires interdisciplinary approaches. Evotec and QUOD researchers have assembled an expert team that efficiently combines domain knowledge in medicine and biology with high-throughput molecular analysis and modern data science. Together, we are exploring the complex relationships between biopsy molecular profiles, donor and recipient clinical phenotypes, and graft outcomes in all major organ recovery and transplant conditions. This exploratory analysis of biopsy transcriptomes involves unsupervised clustering, dimensionality reduction and embedding of clinical data and molecular signatures to enable a data-driven hypothesis generation.
Importantly, the multidimensional characterisation of QUOD biopsy transcriptomes has enormous potential beyond research in organ donation and transplantation, with direct applications in modern drug discovery. First, samples from healthy organ donors that are obtained from the Oxford Transplant Biobank (OTB) can serve as reference for the molecular analysis of other disease-focused prospective cohort studies, which often lack appropriate controls. Second, organ donors often present with early, subclinical disease phenotypes that potentially provide unique insights into the onset and early progression of metabolic and other common diseases. For example, Evotec has integrated a data-driven selection of QUOD and NURTuRE (National Unified Renal Translational Research Enterprise) kidney biopsy transcriptomes ranging from healthy to end-stage chronic kidney disease to model disease initiation and progression at the molecular level. We are also combining transcriptomic analysis with AI-assisted digital pathology to characterise liver biopsies for steatosis, inflammation and fibrosis and determine their position along the spectrum of fatty liver disease.
Ultimately, these systems biology approaches aim to positively impact the lives of patients with organ transplantation, cardiometabolic diseases and related complications by leveraging advanced molecular analysis and interdisciplinary expertise. This transformative work is only possible through close collaboration between academia and industry, combining their strengths and resources to achieve meaningful advances in knowledge that will help improve patient care and therapeutic intervention.