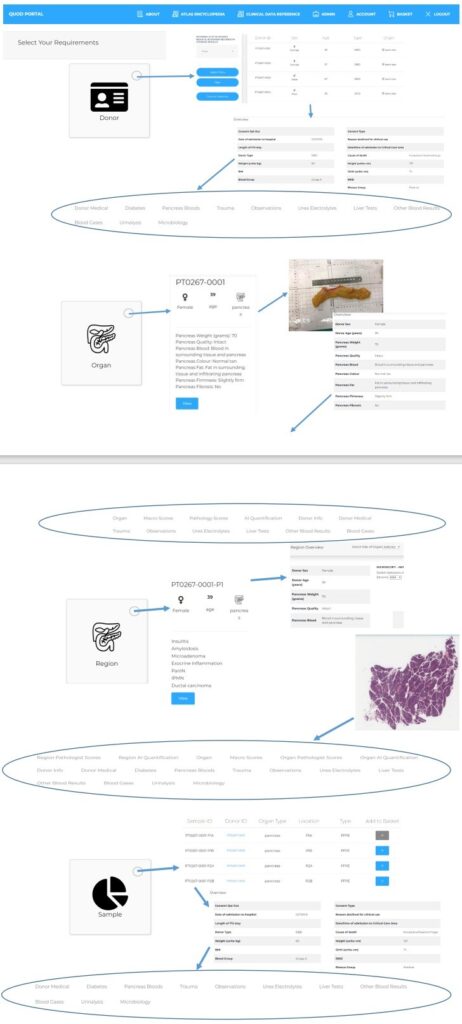
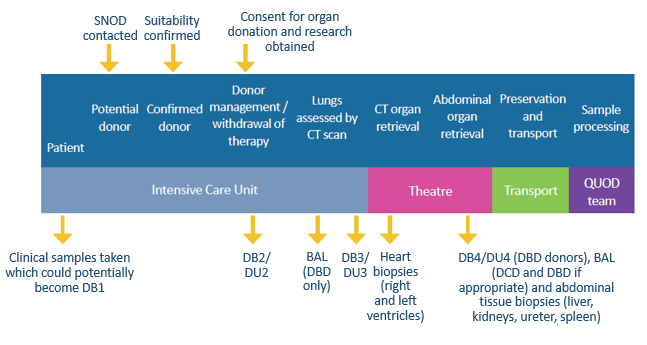
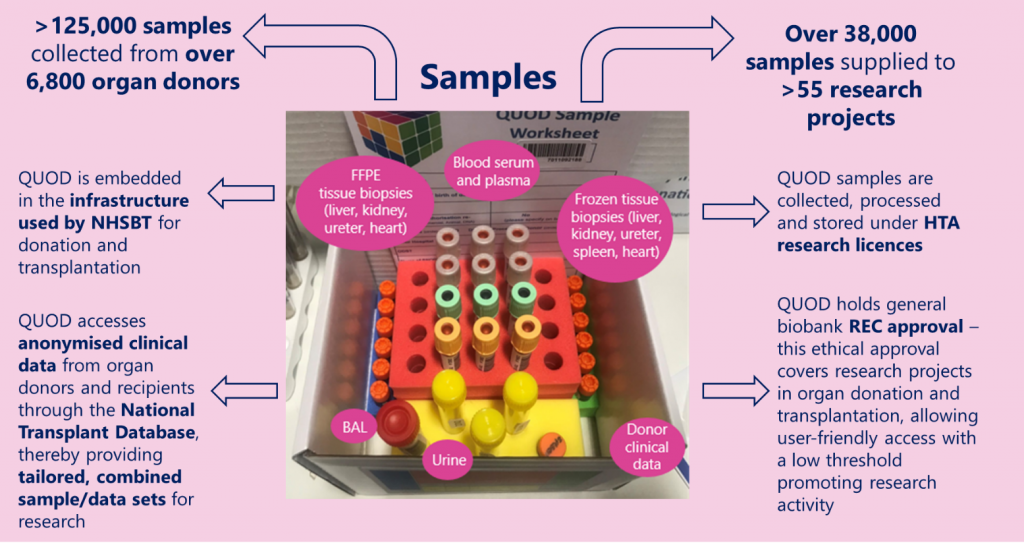
For this issue of the QUOD newsletter, we caught up with Colin Wilson, Professor of Transplantation and Hepato–Pancreato–Biliary (HPB) Surgery at Newcastle University, NHS-employed Consultant Surgeon, and QUOD Principal Investigator for Newcastle. We spoke to Colin about his academic and clinical roles in transplantation as well as his role within the QUOD programme, including how QUOD is supporting the development of a novel artificial intelligence (AI)-informed assessment of organ quality.
In his role as QUOD Principal Investigator for Newcastle, Colin coordinates specimen collection and onward transport to Oxford. He works with various teams within the Newcastle-upon-Tyne Hospitals Trust; the HPB team delivers the QUOD boxes to Oxford and the organ retrieval team underpins this. Colin actively supports the professional development of his team members and encourages their involvement with QUOD.
Colin leads a team at the NIHR Blood and Transplant Research Unit in Organ Donation and Transplantation, which is a collaboration between Newcastle and Cambridge Universities. The programme involves clinical work and registry analyses to look at ways to improve organ utilisation and provide a scientific basis to develop policies in clinical practice around organ utilisation. The programme delivers work packages, protocols, projects, and trials. One such trial is OrQA (Organ Quality Assessment), which, in collaboration with QUOD, is developing a tool that uses AI-driven analysis of photographic images of organs (taken using devices such as smartphones) to predict their suitability for transplantation by assessing factors such as perfusion quality or percentage steatosis (in liver). Colin recalled his experiences as a child using his first camera, the rigmarole of taking the film to be developed, and the wait to receive the prints, whereas nowadays we all have very high-resolution cameras on our phones that could be applied to transform our approach to medicine. His enthusiasm for the use of technology to advance transplantation outcomes is clear and is just one of his visions for the future of transplantation.
Looking ahead, Colin is keen to support medical students aspiring to become transplant surgeons and is actively involved with the North East Surgical Training Academy (NESTAC) training and mentorship programme, which connects students and trainees with mentors. He considers himself to be extremely lucky to be able to support some of the very talented medical students who are interested in careers in transplant surgery. The pathway to becoming a consultant transplant surgeon begins with a one-year laboratory-based MRes project that is written up in a thesis and presented as oral and poster presentations, which may go on to be presented at conferences and meetings, followed by progression to academic foundation doctor (core training level), academic clinical fellow, and finally clinical lecturer. The programme takes around 10 years from start to finish and Colin is very much looking forward to seeing the first person “come off the conveyor belt”, who he first met as a medical student keen to become a consultant transplant surgeon. He has mentored and supported him throughout his career development and says “it is going to be a really big moment that I’m looking forward to”.
Colin’s own journey to become a transplant surgeon began in 1999, when he was working as a Senior House Officer on a gastroenterology ward with Oliver James, Professor of Hepatology, at the Freeman Hospital, Newcastle. It was here that he realised that liver transplantation was key to the successful treatment of many patients with liver ailments. In 2001, he began his PhD, supervised by Prof. David Talbot, on non-heart-beating kidney transplantation, which was novel and challenging work at the time. He then went on to complete his training as a Surgical Registrar from 2006–2012.
While transplant surgery can present challenges, Colin describes his job as rewarding and he is very much future-focused. He is driven by research, developing technology, and building strong teams, and most enjoys creating and delivering novel solutions to overcome (or work around) the hurdles and obstacles facing transplantation. His research aims to identify how to make the best use of the resources available, such as finding ways to overcome non-use of organs from deceased donors and achieve good long-term outcomes for patients, while prioritising safety. He is passionate about supporting the next generation of UK transplant surgeons, who will also face issues for patients with organ failure but may consider newer approaches such as the use of stem cells, AI, or xenotransplantation, which has been approved in the USA but is not currently implemented in the UK.
Colin is unquestionably proud of his hugely dedicated and committed team, comprising particularly talented members, all with broad-ranging areas of expertise and who have won numerous esteemed prizes for their work in transplantation, published cutting-edge research, and are developing “staggering” novel AI work. He mentors his team academically and it is hugely important to him to provide them with an environment in which they can flourish.
Colin describes the QUOD programme as “engaging and outward-looking”, and really values the focus it brings to transplant research. Of particular importance to him are the governance structures in place, in addition to the core biorepository work, as well as the fellowship among like-minded individuals who can build projects on top of the infrastructure that QUOD brings. “It’s not just what QUOD is, but what QUOD has allowed to build around it and the connections that has made it such a valuable resource for UK transplantation.” He believes that the UK is world-leading in many areas of transplantation and QUOD is at the core of that.
In addition to his work in Newcastle, Colin’s job sees him travelling to and presenting at national and international meetings. He will be co-chairing the ESOT Congress 2025 in London, which will focus on sustainability, not just in terms of climate emergency but also workforce and the issues and challenges facing the NHS and European healthcare systems that are struggling to deliver doctors, nurses, and specialists in transplantation.
While his work has taken him to various countries over the years, outside of his busy full-time clinical and academic roles, Colin cherishes time with his family, enjoying activities such as table tennis and travelling together, and is looking forward to a family holiday in the next few weeks.
Interviewed by Jenny Collins.