Q: Which organ donors are included in the QUOD Bioresource?
A: QUOD samples are collected from donors after brain death (DBD) and donors after circulatory death (DCD), including paediatric donors over five years old.
Q: What biological samples are being collected?
A: Blood, urine and tissue samples at a variety of different time points during the donor management period and retrieval operation. The type of samples included in the QUOD collection may be revisited in response to research needs and in line with QUOD ethics approval.
Q: When are samples collected?
A: The samples are taken from organ donors at 4 different time points during the donor management period, up until the point of organ retrieval. Tissue samples are collected, when appropriate, from left and right kidneys, liver, ureter, spleen and heart.
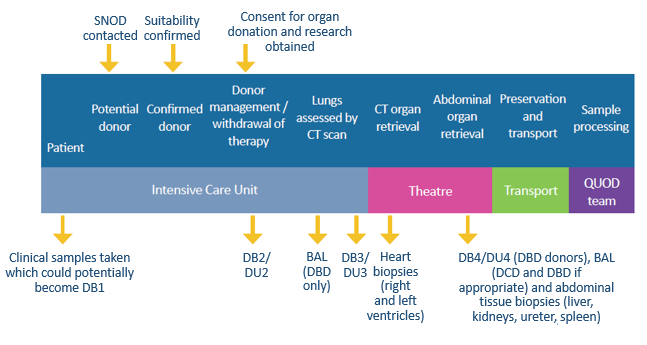
Q: How are the samples processed and stored?
A: Blood, urine and bronchoalveolar (BAL) samples are centrifuged and supernatants are aliquoted into 0.5ml or 0.925ml aliquots at -80°C. The tissue biopsies are halved at the time of collection and stored in formalin and RNAlater.
Upon receipt at the QUOD biobank, the formalin samples are processed into formalin fixed paraffin embedded blocks, allowing a wide range of histological techniques to be exploited. The RNAlater samples are snap frozen in liquid nitrogen and stored at -196°C in vapour phase liquid nitrogen. This allows highly sensitive techniques to be applied, such as –omics technologies (proteomics, metabolomics and transcriptomics).
Q: Why doesn’t QUOD use snap frozen samples?
A: With QUOD obtaining samples from 90% of all deceased donors in the UK where consent was given for donation and research, the distribution and management of liquid nitrogen across all of our donor hospitals in the UK would be complex, costly, and unduly risky! Early in the QUOD project we conducted rigorous audit studies to ensure that RNALater storage still allows for high-quality –omics work. Academic and commercial investigators have been very pleased with the quality and usability of the samples that we have provided.
Q: What are the consequences of taking biopsies?
A: Biopsies are routinely taken in clinical settings. QUOD adheres to strict protocols regarding the biopsy procedure to ensure any potential complications are prevented. Biopsies are taken on the backtable after a period of cold perfusion. To ensure appropriate haemostasis and prevent bleeding after reperfusion at time of transplantation, the retrieval surgeon will place a prolene suture at the biopsy site before packaging the organ for transportation.
Q: How many samples are available?
A: The QUOD biobank has over 95,000 biosamples from over 5,200 organ donors. Click here to see the latest QUOD statistics report.
Q: Who can access QUOD samples?
A: Researchers from any institution or biomedical company around the world can apply for QUOD samples, the proposals are judged on scientific merit. Click here to find out how to apply.
Q: Why does QUOD charge for samples? How are charges determined?
A: QUOD is a not-for-profit programme. Core funding for the biobank infrastructure is provided by NHS Blood and Transport (NHSBT). Since 2017 the NHSBT Executive Board have required QUOD to recover a proportion of its operating budget (10% for 2017-18, rising to 50% by 2023). To reach this goal, QUOD has implemented a modest fee for samples.
QUOD aims to keep the cost for a sample for the individual researcher as low as possible. Each year we review the number of samples distributed in the previous year to make conservative forecasts for the upcoming year, and then divide our cost recovery target figure by the projected number of samples distributed. This generates a ‘cost per biobank item’ that reflects an appropriate percentage of the cost to collect, store, and distribute that biobank item. Each autumn, the Steering Committee reviews and approves a pricing proposal for the upcoming year.
Additionally, QUOD has implemented a three-tier pricing structure. Tier-1 applies to projects by academic researchers relevant to organ donation and transplantation. Tier-2 is for academic research groups not directly involved in donation and transplantation and is 250% of the Tier-1 rate. Tier-3 applies to industrial applicants seeking samples for research and is 400% of the base rate. For 2020, the Tier-1 rate is £12.13 per biobank item.
Industrial applicants considering projects over 1,000 samples, or with interest in longer-term research collaborations, should contact us at quod-research@nds.ox.ac.uk to discuss options
Q: How long will it take to receive my samples from application date?
A: In general, we are able to review and approve Part A applications within one week, and Part B applications within two weeks following the submission to the QUOD Steering Committee. In between those applications, researchers will often be in dialogue with the QUOD team to refine sample and data requests. This process can be quite swift if researchers are clear on what they need, but sometimes extends over a month or more if researchers need more assistance in refining their project plans. Once applications are approved, an MTA must be signed before samples can be released. From that point, the provision of samples depends on the overall workload in the QUOD lab and the size of the sample request, but can be as brief as two weeks in the right conditions. In general, researchers should plan for at least a 3 month release window from the point of submitting the Part A, with larger or more complex projects taking longer.
